Ocular Complications of Diabetes Mellitus1
Lloyd M. Aiello
Lloyd Paul Aiello
Jerry D. Cavallerano
Ocular complications in diabetes are frequent, distressing and destined to become one of the challenging problems of the future.
These prophetic words of Dr. Howard Root opened the chapter on ocular complications in the 1935 edition of Joslin’s The Treatment of Diabetes Mellitus (1). Indeed, as insulin increased the life span of persons with diabetes, diabetic retinopathy became a major cause of severe visual loss in the United States and in other industrialized countries of Europe and the Americas. By the 1960s, diabetic retinopathy was recognized as the leading cause of new, severe visual loss in the United States among persons 21 to 74 years old. Diabetic retinopathy is still neither preventable nor curable.
Dedicated efforts by researchers and patients, however, have established treatment and surgical modalities that can reduce the 5-year risk of severe visual loss (visual acuity 5/200 or worse) from proliferative diabetic retinopathy (PDR) to less than 2% and the 5-year risk of moderate visual loss (a doubling of the visual angle; e.g., 20/20 reduced to 20/40) from diabetic macular edema to 12% or less.
Ongoing research efforts continue to hold promise that diabetic retinopathy eventually will be curable or preventable. Presently, however, clinical goals must concentrate on identifying eyes at risk of visual loss and ensuring that appropriate and timely laser surgery is offered. If patients with diabetes receive currently recommended care, remarkable preservation of vision can be achieved.
SIGNIFICANCE OF APPROACHING/REACHING HIGH-RISK PROLIFERATIVE DIABETIC RETINOPATHY AND CLINICALLY SIGNIFICANT MACULAR EDEMA
In 1969, the first evidence of the effectiveness of scatter (panretinal) laser photocoagulation surgery in the treatment of diabetic
retinopathy was promulgated in the ophthalmologic and medical communities (2). Since these promising beginnings, dramatic strides have been made in treating diabetic retinopathy and macular edema through the effective use of scatter (panretinal) laser and other surgical techniques. The value of these techniques has received strong support from the findings of three major nationwide, randomized, and controlled clinical trials in the United States: the Diabetic Retinopathy Study (DRS) (3,4,5,6,7,8,9,10,11,12,13,14,15,16), the Early Treatment Diabetic Retinopathy Study (ETDRS) (17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39), and the Diabetic Retinopathy Vitrectomy Study (DRVS) (40,41,42,43,44). With proper diagnosis and treatment, the 5-year risk of severe visual loss from PDR could be virtually eliminated. Additionally, clinical trials in the United States (45,46,47,48,49,50,51,52), Japan (53), and the United Kingdom (54,55) have demonstrated that intensive control of diabetes, as measured by glycosylated hemoglobin (HbA1c) levels, can reduce the risk of onset and progression of diabetic retinopathy.
retinopathy was promulgated in the ophthalmologic and medical communities (2). Since these promising beginnings, dramatic strides have been made in treating diabetic retinopathy and macular edema through the effective use of scatter (panretinal) laser and other surgical techniques. The value of these techniques has received strong support from the findings of three major nationwide, randomized, and controlled clinical trials in the United States: the Diabetic Retinopathy Study (DRS) (3,4,5,6,7,8,9,10,11,12,13,14,15,16), the Early Treatment Diabetic Retinopathy Study (ETDRS) (17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39), and the Diabetic Retinopathy Vitrectomy Study (DRVS) (40,41,42,43,44). With proper diagnosis and treatment, the 5-year risk of severe visual loss from PDR could be virtually eliminated. Additionally, clinical trials in the United States (45,46,47,48,49,50,51,52), Japan (53), and the United Kingdom (54,55) have demonstrated that intensive control of diabetes, as measured by glycosylated hemoglobin (HbA1c) levels, can reduce the risk of onset and progression of diabetic retinopathy.
Nevertheless, diabetic retinopathy remains a leading cause of blindness in the United States for persons between the ages of 20 and 74 years (56,57,58). This blindness usually results from nonresolving vitreous hemorrhage, traction retinal detachment, or diabetic macular edema. However, the 5-year risk of severe visual loss can be reduced to less than 2% if a person with diabetic retinopathy approaching or just reaching high-risk proliferative retinopathy, as defined below, undergoes scatter (panretinal) laser photocoagulation surgery (59,60). Furthermore, people with clinically significant diabetic macular edema (CSME) can reduce the risk of moderate visual loss by 50% or more, to approximately 12% or less, if they receive appropriate focal laser surgery (17). Because diabetic retinopathy is often asymptomatic in its most treatable stages, early detection of diabetic retinopathy through regularly scheduled ocular examination is critical.
This chapter reviews prognostic implications of the lesions of diabetic retinopathy and the risks of progression of retinopathy, placing particular emphasis on identifying patients at risk of visual loss and in need of laser surgery. The laser treatment techniques are described in only general terms in this chapter but are carefully detailed in ETDRS reports 3 and 4 (19,20). Nonretinal ocular complications of diabetes mellitus and alterations in visual function also are discussed.
EPIDEMIOLOGY OF DIABETIC RETINOPATHY
An estimated 16 to 17 million Americans have diabetes mellitus (diabetes), but approximately 6 million of these cases have not been diagnosed (61). Among the American population with diabetes, 5% to 10% have type 1 diabetes (insulin-dependent diabetes), which is usually diagnosed before the age of 40 years. The majority of diabetic patients, however, have type 2 diabetes (non-insulin-dependent diabetes), which is usually diagnosed after the age of 40 years; these patients may or may not be treated with insulin. While those with type 1 diabetes experience a high incidence of severe ocular complications and are more likely to develop significant ocular problems during their lifetimes, those with type 2 diabetes account for the majority of clinical cases of diabetic eye disease because of their larger overall number.
Diabetic retinopathy is a highly specific vascular complication of both type 1 and type 2 diabetes, and the duration of diabetes is a significant risk factor for the development of retinopathy (62). After 20 years of diabetes, nearly all patients with type 1 diabetes and more than 60% of those with type 2 diabetes have some degree of retinopathy. Laser surgery and other surgical modalities help minimize the risk of moderate and severe visual loss from diabetes mellitus and, in some cases, restore useful vision for those who have suffered visual loss. These surgical modalities, particularly laser surgery, are most effective if initiated when a person approaches or just reaches high-risk PDR or before a person has lost visual acuity from diabetic macular edema (26).
The 5-year risk of severe visual loss from high-risk PDR may be as high as 60%, and the risk of moderate visual loss from CSME may be as high as 30%. Because PDR and macular edema may cause no ocular or visual symptoms when the retinal lesions are most amenable to treatment, a major clinical goal is to identify eyes at risk of visual loss and ensure that patients are referred for laser surgery at the appropriate time. Even minor errors in diagnosis of the level of retinopathy can result in a significant increase in a person’s risk of visual loss.
Furthermore, collateral health and medical problems present a significant risk for the development and progression of diabetic retinopathy (Table 54.2) (63). These factors include pregnancy (64,65,66), chronic hyperglycemia (45,46,47,48,49,50,51,52,53,54,55,67,68,69,70), hypertension (54,71), renal disease (69), and hyperlipidemia (37,72). Patients with these conditions require careful medical evaluation and follow-up for the progression of diabetic retinopathy and optimization of their medical status.
TABLE 54.2. Medical Problems Presenting Significant Risk for Development of Diabetic Retinopathy or Affecting Its Course | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
CLINICAL TRIALS OF DIABETIC RETINOPATHY: SCIENTIFIC BASIS FOR MANAGEMENT
In the United States, the results of four nationwide randomized clinical trials have determined in large part the strategies for appropriate clinical management of patients with diabetic retinopathy. The United Kingdom Prospective Diabetes Study (UKPDS) adds significant information to supplement the findings of the studies in the United States.
Diabetic Retinopathy Study
The DRS (3,4,5,6,7,8,9,10,11,12,13,14,15,16) (Table 54.3) conclusively demonstrated that scatter (panretinal) photocoagulation significantly reduces the risk of severe visual loss from PDR, particularly when high-risk PDR is present.
TABLE 54.3. Diabetic Retinopathy Study | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 54.1. Abbreviations of Commonly Used Terms | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Early Treatment Diabetic Retinopathy Study
The ETDRS (17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39) provided valuable information concerning the timing of scatter (panretinal) laser surgery for advancing diabetic retinopathy and conclusively demonstrated that focal photocoagulation for CSME reduces the risk of moderate visual loss by 50% or more (Table 54.4). Furthermore, the ETDRS demonstrated that both early scatter (panretinal) laser surgery (before high-risk PDR) and deferral of treatment “until and as soon as high-risk PDR developed” are effective in reducing the risk of severe visual loss. Scatter laser surgery, therefore, should be considered as an eye approaches the high-risk stage and “usually should not be delayed if the eye has reached the high-risk proliferative stage” (26). For patients with type 2 diabetes mellitus or type 1 diabetes mellitus of long standing, early photocoagulation should be considered.
TABLE 54.4. Early Treatment Diabetic Retinopathy Study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Diabetic Retinopathy Vitrectomy Study
The DRVS (40,41,42,43,44) provided guidelines for the most opportune time for vitrectomy surgery for patients with type 1 and type 2 diabetes who suffered from vitreous hemorrhage (40,41,44) or from severe PDR in eyes with useful vision (Table 54.5) (42,43). In these early years of pars plana vitrectomy, early vitrectomy for eyes with recent severe vitreous hemorrhage and a visual acuity of less than 5/200 was beneficial, especially for patients with type 1 diabetes. Furthermore, the chance of achieving visual acuity of 10/20 or better was increased by early vitrectomy in eyes with severe proliferating neovascular retinopathy, again especially for patients with type 1 diabetes. However, surgical tools and techniques for vitrectomy in patients with diabetic retinopathy have evolved significantly over the past two decades, making precise prediction of clinical outcome for the DRVS data potentially less applicable and present surgical outcomes probably more favorable.
TABLE 54.5. Diabetic Retinopathy Vitrectomy Study | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Diabetes Control and Complications Trial
The Diabetes Control and Complications Trial (DCCT) (45,46,47,48,49,50,51,52) conclusively demonstrated that intensive control of glycemic levels, as reflected by measurements of HbA1c, significantly reduces the risk of onset of diabetic retinopathy, progression of preexisting retinopathy, and the need for laser surgery for persons with type 1 diabetes. Intensive therapy reduced the risk of
onset of retinopathy by 76% and resulted in a 63% reduction in the risk of progression of retinopathy. There was a 47% reduction in the risk of developing severe nonproliferative diabetic retinopathy (NPDR) or PDR, a 23% reduction in the risk of developing clinically significant macular edema, and a 56% reduction in the risk of requiring laser surgery for those on intensive therapy. Significantly, these benefits persisted for 4 years after the period of intensive control, despite convergence of glycemic control for both groups after 4 years (52). Similar reduction of risks was identified for other microvascular complications of diabetes, such as renal disease and neuropathy.
onset of retinopathy by 76% and resulted in a 63% reduction in the risk of progression of retinopathy. There was a 47% reduction in the risk of developing severe nonproliferative diabetic retinopathy (NPDR) or PDR, a 23% reduction in the risk of developing clinically significant macular edema, and a 56% reduction in the risk of requiring laser surgery for those on intensive therapy. Significantly, these benefits persisted for 4 years after the period of intensive control, despite convergence of glycemic control for both groups after 4 years (52). Similar reduction of risks was identified for other microvascular complications of diabetes, such as renal disease and neuropathy.
United Kingdom Prospective Diabetes Study
The UKPDS (54,55) and studies in Japan (53) demonstrated similar reduction in risk for onset and progression of diabetic retinopathy for persons with type 2 diabetes. The UKPDS enrolled 3,867 patients with newly diagnosed type 2 diabetes. Intensive therapy to control blood glucose, using either sulfonylureas or insulin, resulted in a 17% risk reduction for progression of diabetic retinopathy, a 29% risk reduction in the need for laser photocoagulation surgery, a 23% risk reduction for the development of vitreous hemorrhage, and a 16% risk reduction in legal blindness.
DIAGNOSIS, CLASSIFICATION, AND MANAGEMENT OF DIABETIC RETINOPATHY
Retinal Lesions
The processes by which diabetes mellitus results in retinopathy and maculopathy are not fully understood. It is apparent in studies with laboratory animals, however, that hyperglycemia itself, even in animals not genetically diabetic, is sufficient to cause diabetic retinopathy (73). The elevated blood glucose level is thought to induce structural, physiologic, and hormonal changes that affect the retinal capillaries. The retinal capillary mural cells become less functional and endothelial cell dysfunction results (74,75,76). Alteration in retinal blood flow is observed early in the course of diabetes (77,78).
Basic pathophysiologic processes in the development of diabetic retinopathy include (a) loss of pericytes associated with retinal capillaries, (b) thickening of the basement membrane, (c) changes in retinal blood flow, (d) outpouching of capillary walls to form microaneurysms, (e) closure of retinal capillaries and arterioles leading to retinal nonperfusion, (f) breakdown of the blood/retinal barrier with increased vascular permeability of retinal capillaries, (g) proliferation of new retinal and/or iris vessels, (h) development of fibrovascular tissue, and (i) contraction of vitreous and fibrous proliferation with subsequent vitreous hemorrhage and/or retinal detachment as a result of traction.
HEMORRHAGES AND/OR MICROANEURYSMS
The various diabetic retinal lesions and their severity, both alone and in aggregate, are excellent predictors of the risk of progression of retinopathy and visual loss (Table 54.6) (29). The development of the various lesions of diabetic retinopathy is believed to result from the occurrence of various pathologic processes and interactions during the course of diabetes and development of diabetic eye disease. The retinal pericytes, which are intimately associated within the basement membrane of the retinal endothelial cells, are normally present in a one-to-one ratio with the endothelial cells themselves. This is a ratio higher than that found anywhere else in the body. This finding and other cell-culture data have suggested that the retinal pericytes are critical supporting cells for the retinal capillaries (79,80). The loss of the retinal pericytes is thought, therefore, to be a factor contributing to the development of endothelial cell dysfunction and weakness of the retinal capillary wall, possibly contributing to the formation of microaneurysms, which, along with venous dilatation, is one early clinical sign of diabetic retinopathy (15,19,20).
TABLE 54.6. Proliferative Diabetic Retinopathy at 1-Year Visit, By Severity of Individual Lesion | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
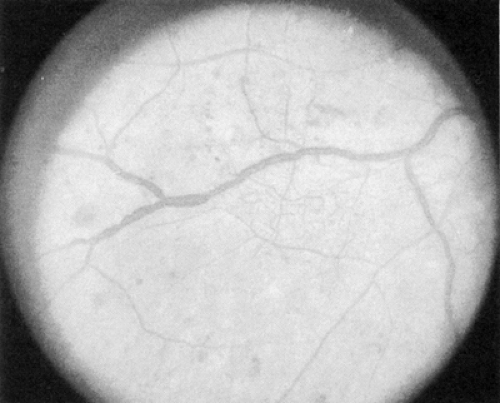
The early clinical signs of diabetic retinopathy are micro-aneurysms, which are saccular outpouchings of retinal capillaries (Fig. 54.1). Ruptured microaneurysms, leaking capillaries, and intraretinal microvascular abnormalities can result in intraretinal hemorrhages. The clinical appearance of these hemorrhages reflects the retinal architecture at the retinal level of the hemorrhage. Hemorrhages in the nerve-fiber layer assume a more flame-shaped appearance, coinciding with the structure of the nerve-fiber layer that runs parallel to the retinal surface. Hemorrhages deeper in the retina, where the arrangement of cells is approximately perpendicular to the surface of the retina, assume a pinpoint or dot shape and are more characteristic of diabetic retinopathy.
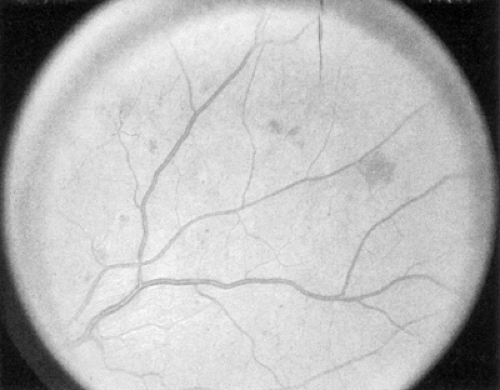 Figure 54.1. Standard photograph 2A of the modified Airlie House classification of diabetic retinopathy demonstrating a moderate degree of hemorrhage and/or microaneurysms. |
The term “dot/blot hemorrhages” has been used to describe these small intraretinal hemorrhages characteristic of diabetic retinopathy. Because it is very difficult, if not impossible, to distinguish small dot/blot hemorrhages from microaneurysms, and because critical evaluation of these lesions has determined that little additional clinically significant information is obtained by differentiating these two lesions, they are classically evaluated together and referred to as “hemorrhages and microaneurysms.”
VENOUS CALIBER ABNORMALITIES
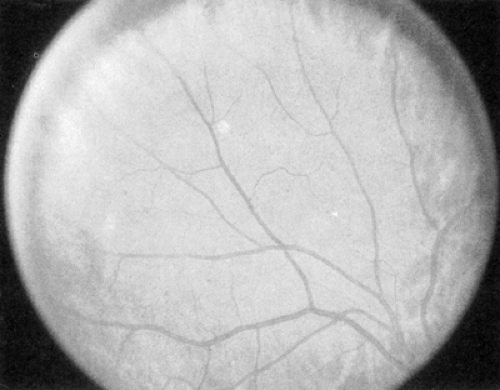
Venous caliber abnormalities (Fig. 54.2) are early indicators of diabetic retinopathy and are often indicators of severe retinal hypoxia. These abnormalities can be venous dilatation, beading, or loop formation. These venous changes usually occur where there are large areas of nonperfusion adjacent to the veins. Treatment with scatter (panretinal) photocoagulation may cause these abnormal veins to become more normal in appearance over time.
INTRARETINAL MICROVASCULAR ABNORMALITIES
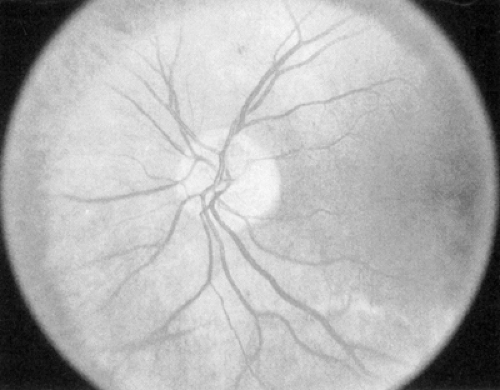
Intraretinal microvascular abnormalities (IRMAs) (Fig. 54.3) are preexisting vessels with endothelial cell proliferation that some consider new vessel growth within the retina that become “shunts” through areas of nonperfusion. IRMAs may be seen adjacent to cotton-wool spots, which result from microinfarcts in the nerve-fiber layer of the retina. IRMAs are often associated with a severe stage of nonproliferative retinopathy, and frank
neovascularization is likely to appear on the surface of the retina or optic disc within a short time.
neovascularization is likely to appear on the surface of the retina or optic disc within a short time.
RETINAL NEOVASCULARIZATION
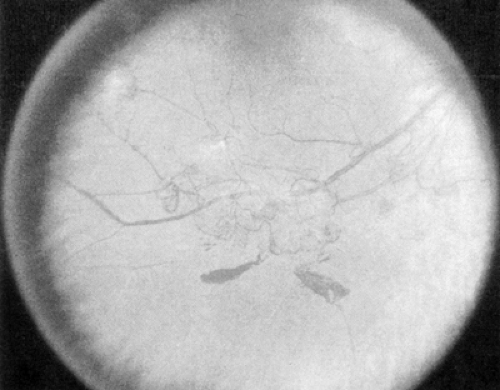
PDR (Fig. 54.4) is marked by the development of new, abnormal retinal vessels. The rate of growth of these new vessels is variable. These vessels grow either at or near the optic disc [neovascularization of the disc (NVD)] or elsewhere on the surface of the retina [neovascularization elsewhere (NVE)]. Translucent fibrous tissue is often associated with the new vessels. This fibroglial tissue appears opaque and becomes adherent to the adjacent vitreous.
Patients with high-risk PDR require immediate scatter laser photocoagulation. High-risk PDR is characterized by any one or more of the following lesions: (a) NVD approximately one fourth to one third the disc area or more in size (i.e., greater than or equal to NVD in ETDRS standard photo 10A) (9,27) (Fig. 54.4); (b) NVD less than one fourth the disc area in size if fresh vitreous or preretinal hemorrhage is present; or (c) NVE greater than or equal to one half the disc area in size if fresh vitreous or preretinal hemorrhage is present (Fig. 54.5). Because identification of high-risk characteristics is critical for determining delivery of sight-saving care, careful attention must be paid to the presence or absence of new vessels, the location of new vessels, the severity of new vessels, and the presence or absence of preretinal or vitreous hemorrhage (5).
Levels of Diabetic Retinopathy
Scatter (panretinal) laser photocoagulation surgery should be considered as retinopathy approaches or reaches the high-risk stage of PDR. An eye is considered to be approaching the high-risk stage when there are retinal signs of severe or very severe NPDR or new vessels not fulfilling the definition of high-risk PDR (see Proliferative Diabetic Retinopathy), especially if associated with very severe NPDR. The baseline level of retinopathy
is a strong indicator of the risk of progression from the NPDR stage to both early PDR and high-risk PDR (Tables 54.6, 54.7, 54.8) (29).
is a strong indicator of the risk of progression from the NPDR stage to both early PDR and high-risk PDR (Tables 54.6, 54.7, 54.8) (29).
TABLE 54.7. Levels of Retinopathy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 54.8. General Management Recommendations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree