and Lisa Diller2
(1)
Pediatric Hematology, Oncology and Stem Cell Transplantation, University of Chicago, Comer Children’s Hospital, Chicago, IL, USA
(2)
Pediatric Oncology, Dana-Farber Cancer Institute/Harvard Medical School, Boston, MA, USA
17.2.3 Breast Hypoplasia Detection
17.2.4 Breast Hypoplasia Management
17.3.2 Lactation Issues Management
17.4.3 Breast Cancer Genetics
17.4.5 Prevention Strategies
Impairment of breast development, disruption of breast function, and breast cancers are all late consequences that have been observed in survivors of childhood cancer. Normal breast development can be impaired by exposure to radiation, prior surgery, and by decreased estrogen exposure associated with therapy-induced ovarian or hypothalamic disruption. Normal function of the breast as the lactation organ has not been well studied in survivors. Most studies have focused on breast cancer risks in survivors of childhood cancer, with a particular concern for breast cancers after radiation to the chest. Recent work has focused on surveillance strategies, prophylaxis, and changes in primary cancer therapy that might reduce the risk of secondary breast cancer. Finally, understanding of the underlying genetic risk factors for secondary breast cancers may determine future strategies for prevention of breast malignancy after childhood cancer. In this chapter, we review normal breast development, risk factors for secondary breast cancer, surveillance, and prevention strategies, as well as what is known about management of breast hypoplasia and lactation impairment in survivors.
17.1 Brief Overview of Normal Development
The mammary glands consist of a branching network of ducts ending in buds, which during puberty evolve into increasing numbers of alveolar lobules. During pregnancy and lactation, these lobules become secretory. In human embryonic development, the first sign of mammary glands are found in the form of a band-like thickening of epidermis, the mammary line or ridge. This extends on each side of the body from the base of the forelimb to the region of the hind limb by the seventh week of gestation. Most of the mammary line disappears quickly, but a small portion in the thoracic region persists and penetrates the underlying mesenchyme, forming the breast bud. The bud sprouts 16–24 cords, which ultimately form the lactiferous ducts surrounded by the alveoli of the gland. The ducts at first open into a small epithelial pit in the bud, but shortly after birth, this pit matures into the nipple by proliferation of the underlying mesenchyme [1]. At birth, the breast of both the male and female is identical. At puberty the female breast bud enlarges, followed by development of the mammary glands and, subsequently, deposition of fat. The nipple and areola enlarge as well [2]. These developmental changes are determined by circulating levels of female pituitary and gonadotropic hormones; specifically, estrogen generates ductal growth, progesterone stimulates alveolar lobules, prolactin stimulates the alveoli to produce milk products, and oxytocin then stimulates the letdown and release of milk [3].
17.2 Breast Hypoplasia After Childhood Cancer
17.2.1 Breast Hypoplasia Due to Radiation Exposure
In the growing breast, the most sensitive structure is the breast bud. As little as 10 Gy to the breast bud will cause breast hypoplasia (underdevelopment); doses above 20 Gy may ablate development altogether [4]. In patients treated with pulmonary irradiation for Wilms’ tumor, four out of ten females had hypoplastic breast development, including two who received less than 20 Gy [5].
17.2.2 Breast Hypoplasia as a Consequence of Ovarian Failure
A consequence of premature ovarian failure prior to or in the midst of puberty due to exposure to alkylator chemotherapy, radiation involving the pelvis and/or surgery requiring removal of the ovaries, is the failure of breast development. Survivors with primary ovarian failure will not go through puberty without hormone replacement therapy (HRT). Decisions around timing and dosage of HRT need to be individualized for the patient, considering chronological age, stage of secondary sexual characteristics, as well as psychological issues, such as desire to match peers with respect to pubertal development [6]. It should also be recognized that there is a fine balance which needs to be achieved when prescribing estrogen replacement in girls with premature ovarian failure – while at low doses it stimulates longitudinal growth, at higher doses it can result in premature epiphyseal closure and ultimately reduction in adult height. Thus, initiation of HRT should take into account maximizing longitudinal growth. In the absence of any breast development, it has been recommended by age 12 years; girls with known ovarian failure should start low-dose estrogen to stimulate breast development [6]. After about 6–18 months of this initial therapy, based on the amount of breast development prior to initiating HRT and the desired rate of progressing through puberty, the estrogen dose should be increased. Once breast development is complete (usually after 12–24 months of the higher-dose estrogen treatment), cyclic dosed progestins [such as combined estrogen-progestin products, such as oral contraceptive pills (OCPs), or transvaginal or transdermal formulations] can be incorporated to the HRT in order to maintain the health of the endometrium. Progesterone does not play a significant role in breast development and early puberty. In order to mimic “typical” puberty, it is considered most appropriate to begin HRT with estrogen monotherapy.
17.2.3 Breast Hypoplasia Detection
In females who were exposed to over 10 Gy to the breast (including chest, whole-lung, mediastinal, axilla, mini-mantle, mantle, extended mantle, total-body irradiation), in particular those who were treated when they were prepubertal, yearly breast exams should be performed to identify failure of breast development [7].
17.2.4 Breast Hypoplasia Management
Breast hypoplasia may be corrected by breast augmentation. Upon completion of growth and puberty, females may be referred for surgical consultation for breast reconstruction. Optimally, surgeons should be familiar with hypoplasia in survivors as healing may be impaired in previously irradiated areas. Providers should consider the survivors’ emotional and psychological status when determining this referral [7]. A downside of the procedure is that breast augmentation may make breast cancer surveillance more difficult, though studies to date suggest that breast cancer stage at diagnosis and subsequent outcomes are similar in women with and without previous breast augmentation [8].
17.3 Lactation After Childhood Cancer
The functioning of the mammary glands is dependent on an interplay of neuroendocrine factors, as well as health of breast tissue, skin, and glands. Prolactin, produced in the anterior pituitary, is necessary for milk production, and oxytocin, produced in the posterior pituitary, controls milk letdown and ejection. Disruption in the hypothalamic-pituitary axis known to result from cancer therapies, such as cranial radiation for central nervous system tumors or leukemia, may result in dysfunctional lactation [9]. Other therapies that can interfere with the normal anatomy of the breast can also interfere with the ability to successfully lactate. For example, chest radiation or surgeries resulting in hypoplasia or asymmetry can result in decreased ability to successfully lactate. McCullough and colleagues reported a cross-sectional survey study of 81 survivors of childhood and young adult Hodgkin lymphoma, who received chest radiotherapy at a median dose of 41 Gy (range: 27–46 Gy), and found 57 out of 94 (61 %) breast-feeding attempts were reported as successful as compared to 74 of 94 (79 %) attempts in sibling controls [10]. While these findings were encouraging, the researchers did note that lactation compromise was a previously unreported late effect of Hodgkin lymphoma therapy.
17.3.1 Lactation Issues Detection and Surveillance
In patients who were exposed to radiation of the hypothalamic-pituitary axis, patients should be counseled about potential lactation dysfunction. Yearly screening with LH, FSH, prolactin, GH, TSH, and T4 may indicate that lactation dysfunction may be an issue. In females who received radiation to the breast, particularly in those who received over 40 Gy radiation, providers should counsel them that there is an increased risk of lactation failure upon childbearing. However, providers should stress to survivors the health benefits of breast-feeding in order not to dissuade them from attempting breast-feeding.
17.3.2 Lactation Issues Management
For women with hypothalamic-pituitary axis damage, hormone replacement in the case of central hypothyroidism or central GNRH failure may correct inability to lactate. For women exposed to chest radiation, there is not a known treatment for women unable to lactate. Like the general population, these women should be counseled to follow up with their obstetrician, pediatrician, and/or a lactation consultant in the event there is a correctable latch issue or other correctable issue not related to the previous cancer therapy.
17.4 Breast Cancer After Childhood Cancer
Adult survivors of childhood cancer are at an increased risk of breast cancer due to a complex interplay of constitutional factors, exposures through treatment with chemotherapy and radiation, and genetic predisposition [11–14]. Given the significant morbidities and mortality associated with a breast cancer diagnosis, especially given survivors’ previous disease and treatment exposures, it is imperative that health-care providers understand the risks, the biology and genetics, the recommended surveillance guidelines for early detection, and potential prevention strategies available for care of survivors at increased risk for breast cancer.
17.4.1 Risk of Breast Cancer in Childhood Cancer Survivors
Women who were treated with chest radiation therapy for a childhood, adolescent, or young adult malignancy are at significantly increased risk of developing breast cancer at a young age [11, 13, 14]. Hodgkin lymphoma survivors treated with high-dose mantle radiation (over 40 Gy) carry the highest risk of developing breast cancer, though risk is quite high in those who received 30–35 Gy [13, 14]. However, the risk is also significantly elevated in women who received moderate-dose radiation for other childhood and young adult malignancies, such as Wilms’ tumor, non-Hodgkin lymphoma, neuroblastoma, and sarcomas [12, 13]. In light of the improved cure rates for pediatric and young adult cancers over the past several decades, it is estimated that there are currently 50,000–55,000 women in the United States at high risk for breast cancer due to earlier treatment with moderate- to high-dose chest radiation (≥20 Gy) [15–17].
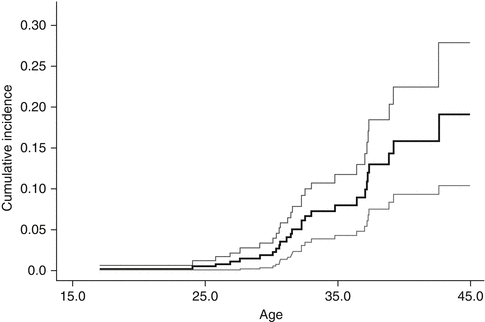
A systematic review [18] of studies focused on breast cancer in women treated with chest radiation for a pediatric or young adult cancer showed significant increased risk of breast cancer, with standardized incidence ratios (SIR) ranging from 13.3 to 55.5 and the absolute excess risks ranging from 18.6 to 79.0 per 10,000 person-years [12, 19–23]. Risk increased as early as 8 years following chest radiation and did not plateau with increasing length of follow-up [12, 19, 24–26]. The cumulative incidence of breast cancer by 40–45 years of age was 13–20 % (12–26 % by 25–30 years of follow-up) [12, 19, 23, 26] (see Fig. 17.1). This incidence is similar to that in women with a BRCA gene mutation, where the cumulative incidence ranges from 10 % to 19 % by age 40 [27–29]. In comparison, the cumulative incidence of invasive breast cancer is 1 % by age 45 in the general population [18].
Radiation dose modifies the risk of breast cancer in this population. Travis and colleagues estimated that among women diagnosed with Hodgkin lymphoma at age 15 years and counseled for screening at the age of 25, 9.2 % of those treated with 20–39 Gy and 11.1 % of those treated with ≥40 Gy would develop breast cancer by age 45 [30]. Inskip and colleagues examined the women with breast cancer in the North American cohort, the Childhood Cancer Survivor Study (CCSS), and found a linear radiation dose-response (see Fig. 17.2), reaching an odds ratio (OR) of 10.8 (95 % CI, 3.8–31) for 40 Gy compared to no radiation exposure [13]. De Bruin and colleagues found that a reduced volume of radiation was associated with decreased risk of breast cancer in Hodgkin survivors [31]; mantle field radiation (cervical, supraclavicular, axillary, mediastinal nodes) was associated with a 2.7-fold increased risk (95 % CI, 1.1–6.9) as compared with similarly dosed (36–44 Gy) mediastinal radiation alone (see Fig. 17.3).
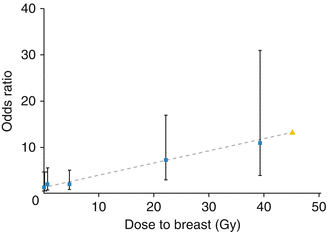
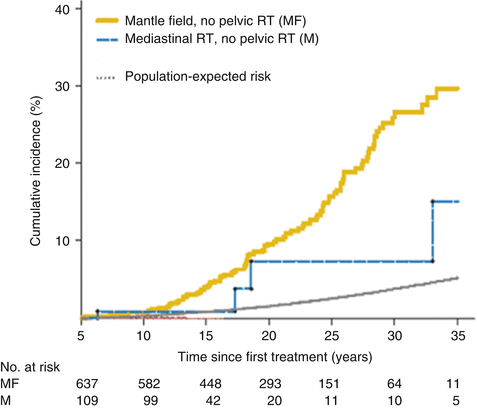

Fig. 17.2
Breast cancer risk by radiation dose to the breast (Permission requested for use from ASCO/JCO, Inskip et al. [13])

Fig. 17.3
Breast cancer risk in Hodgkin lymphoma survivors exposed to mantle and mediastinal fields as compared to the general population (From De Bruin et al. [31])
In the 1980s, the field and dose of radiation used to treat Hodgkin lymphoma was reduced, and therapy was combined with chemotherapy in response to other late effects, such as growth deformities, infertility, and cardiovascular disease. In the most modern pediatric Hodgkin trials, physicians are continuing to examine how to further minimize chest radiation in this population. Ultimately, the breast cancer risk (along with other late effects risks) is not yet known in the more recent large cohorts of Hodgkin survivors treated with lower-dose radiation. However, one report of 112 childhood Hodgkin lymphoma survivors treated with chemotherapy and lower-dose 15–25.5 Gy involved-field radiation described an elevated excess risk of second malignant neoplasms (SMNs) similar to that observed in earlier cohorts [32]. However, the irradiated volume in that study appears to have been more extensive than used in more recent treatment cohorts.
While chest radiation appears to be the most influential factor in a childhood cancer survivor’s risk of breast cancer, other factors have been identified which appear to modify that risk. A family history of breast cancer or sarcoma modestly increases the likelihood women exposed to chest radiation will develop breast cancer [12, 33]. In fact, in Kenney and colleagues’ CCSS study, almost one quarter of the women with early-onset breast cancer in the cohort had not been exposed to chest radiation, suggesting a genetic predisposition to breast cancer [12]. Estrogen exposure may modulate risk, as male breast cancer after radiation is rare. Similarly, in women exposed to breast radiation and ovarian radiation, the risk of breast cancer is substantially reduced compared to women who received chest radiation without gonadal radiation [12, 13, 23, 31, 34]. Finally, high-dose alkylating agent chemotherapy reduces the risk of breast cancer by inducing premature ovarian failure and thereby lowering endogenous estrogens [12, 23, 30, 34].
Earlier studies have indicated that women exposed to chest radiation prior to puberty have lower risk of breast cancer than those treated adolescence [35]. However, Yasui and colleagues identified a methodological issue in the analysis of second malignant neoplasms which did not account for the natural age-associated increase of risk [36]. More recent studies in this population, which incorporate extended years of follow-up and thus account for this natural age-associated risk, have not shown a difference in breast cancer risk among females treated with chest radiation prior to puberty compared with those treated in adolescence [12, 13, 19].
17.4.2 Breast Cancer Biology and Clinical Characteristics
In women treated with chest radiation before the age of 20, the median age of initial breast cancer diagnosis is 32–35 years [12, 26]. Risk begins to increase as early as 8 years following chest radiation exposure. Of note, the median age of diagnosis for breast cancer in the general population is 61 years of age, with 1.9 % of cases occurring between ages 20–34 and 10.6 % between ages 35 and 44 [17].
The majority (77–85 %) of breast cancers in childhood, adolescent, and young adult cancer survivors are characterized by invasive ductal carcinoma, which is comparable to the proportion of invasive ductal carcinomas among breast cancer cases in the general population [37, 38]. In a case-control study of 253 women with breast cancer following Hodgkin lymphoma compared with 741 women with sporadic breast cancer who were matched for age, race, and age at breast cancer, there were no statistically significant differences between groups in histology (including DCIS, invasive ductal carcinoma, and invasive lobular carcinoma), estrogen receptor status, HER2 status, or proportion of patients with multifocal disease [38].
The risk of bilateral breast cancer is increased in women treated at young age with chest radiation. Henderson et al. found, reviewing three studies of Hodgkin lymphoma survivors, that of 219 women with breast cancer, 12.8 % had bilateral disease: 5.5 % synchronous and 7.3 % metachronous [18, 39–41]. This is contrasted to studies of breast cancer in the general population where 3–5 % of cases show bilateral disease, about half of which are synchronous and half metachronous [39–41]. Lastly, while Elkin and colleagues found in their case-control study of breast cancer in Hodgkin survivors as compared to sporadic breast cancer a significantly increased rate of bilateral breast cancer, the overall incidence was lower than in previous studies – 6 % of Hodgkin survivors presented with bilateral disease as compared with 2 % of controls [38]. It is important to note that the average age of childhood cancer survivors at risk for breast cancer following chest radiation is relatively young, so the percent of cases with metachronous disease will likely increase over time.
17.4.3 Breast Cancer Genetics
To date, there is no single gene that has been determined to account for most breast cancer after childhood cancer. Genetic predisposition to cancer can be classified into two categories: genes that are highly penetrant and result in a high risk to individual carriers and low-penetrant genes which may more broadly increase risk to a lesser degree across a population. The genetic predisposition to breast cancer after childhood cancer may involve both types of genes. Nonetheless, several defined cancer family syndromes with known cancer predisposition genes include both childhood cancers and breast cancer.
Li-Fraumeni syndrome (LFS) is a familial cancer syndrome associated with a germline mutation of the TP53 gene [42–44]. A family with a child with a sarcoma whose mother has developed very early-onset breast cancer is perhaps pathognomonic for germline TP53 mutation [45]. However, pedigrees of LFS families are characterized by children with a wide variety of tumor types including not only bone and soft tissue sarcomas, but also brain tumors, adrenocortical carcinomas, leukemias, and other rare tumors. Young women with a history of nearly any diagnosis of cancer as a child should be considered at risk for breast cancer, and care should include querying family history as well as consideration of genetic counseling. Genetic testing for germline TP53 mutations may result in a diagnosis of LFS in these survivors and may inform breast cancer prevention and screening strategies for these survivors and their female relatives [46]. LFS should be suspected in survivors of childhood cancers who subsequently develop breast cancer as young adults and in those rare patients who develop primary breast cancer during adolescence. Although a genetic mutation may be the major underlying factor in developing breast cancer in survivors with LFS, radiation in the field of the first cancer may also further increase the risk of developing breast cancer [47].
Evaluation of the two major familial breast cancer genes, BRCA1 and BRCA2, is now a routine for patients with early-onset breast cancer or strong family history of breast cancer. In a study of a large cohort of BRCA1 and BRCA2 breast cancer families, there did not appear to be an excess of childhood cancers associated with either gene [48]. However, Magnussen et al. demonstrated a substantial risk of childhood cancers in BRCA2 but not BRCA1 families [49]. Biallelic mutations of BRCA2 are associated Fanconi anemia (FA), but have also been reported in Wilms’ tumor patients as well as patients with CNS tumors, some of whom may not have the classic Fanconi features [50, 51]. While these patients are rare and data on FA survivors is lacking (as there are few long-term survivors of FA), the strong association of BRCA2 in the heterozygous form with breast cancer would suggest that survivors of childhood cancers who carry germline biallelic BRCA2 mutations would also carry a high risk of breast cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree