Fig. 1
Different mechanisms of intracellular action of antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs). a Mechanism of action for ASOs, which bind complementary mRNA and cause inhibition of translation or recruit RNase H to cleave the RNA moiety within an RNA–DNA duplex; b Mechanism of action for siRNAs, which includes formation of the RNA-induced silencing complex (RISC) and subsequent degradation of target mRNA
1.2 The RNA Interference (RNAi) Pathway
Fire and Mello first reported the discovery of RNAi-based gene silencing in Caenorhabditis elegans in 1998. This discovery later earned them the Nobel Prize in Physiology or Medicine in 2006 [11]. The RNAi pathway was then established in mammalian cells by Tuschl and colleagues, prompting rigorous development of RNAi-based therapeutics to battle diseases previously considered “undruggable” by traditional pharmaceutical approaches (i.e., small molecules and biotherapeutic antibodies) [12–15]. The RNAi pathway is activated by the presence of dsRNA in the cytoplasm (Fig. 1b) [16–18]. Dicer, a cytoplasmic endoribonuclease, cleaves longer dsRNA into small interfering RNA (siRNA) or microRNA (miRNA) segments, which are typically 21–23 nucleotides in length. The “antisense” or “guide” strands of siRNA or miRNA segments are then recognized and loaded into the RNA-induced silencing complex (RISC), while the “sense” or “passenger strands” are degraded. This activates the RISC complex, leading to Watson-Crick base pairing of complementary target mRNA. Once the target mRNA is bound, its expression can be modified by distinct mechanisms, depending on the biological context. In siRNA-based RNAi, Argonaute 2 (Ago2, an RNA endonuclease in the RISC complex) subsequently cleaves the target mRNA, thereby inhibiting its translation. The RISC complex is recycled and thus cleaves mRNA continuously, resulting in persistent knockdown lasting between 3 and 7 days in dividing cells and up to 3–4 weeks in nondividing cells [19]. In miRNA-based RNAi, multiple mechanisms of silencing are possible, including repression of protein translation and/or deadenylation of target mRNA subsequently leading to its degradation [7, 20].
1.3 Challenges for Oligonucleotide Drug Delivery
Despite these cellular mechanisms that allow for highly specific therapeutic manipulation of genetic expression, a number of barriers to effective delivery are encountered when nucleic acids are systemically injected, limiting their utility in vivo. Unmodified oligonucleotides experience rapid renal clearance, are subject to cleavage by RNases and DNases in serum, and display inefficient uptake by target tissues. Additionally, unmodified oligonucleotides do not efficiently cross the cell membranes and have been shown to trigger a cellular immune response [21–23]. These in vivo barriers to oligonucleotide delivery have slowed the translation of nucleic acid-based therapeutics to the clinic and mandated the use of oligonucleotide-carrier systems, such as polymers/polyplexes [24, 25], dendrimers [26], and lipids [27, 28]. Notably, each of these systems has their own safety concerns and delivery limitations [29]. Nanomaterial-based systems have emerged as potential therapeutic agents for delivering oligonucleotides to cells, and early experiments have shown their great promise [30–32]. Among nanomaterials, spherical nucleic acids (SNAs; Fig. 2) represent an attractive class of single-entity agents, where therapeutic oligonucleotides that can be designed and synthesized to function through either the RNAi or antisense pathway; and these structures are the focus of this chapter.
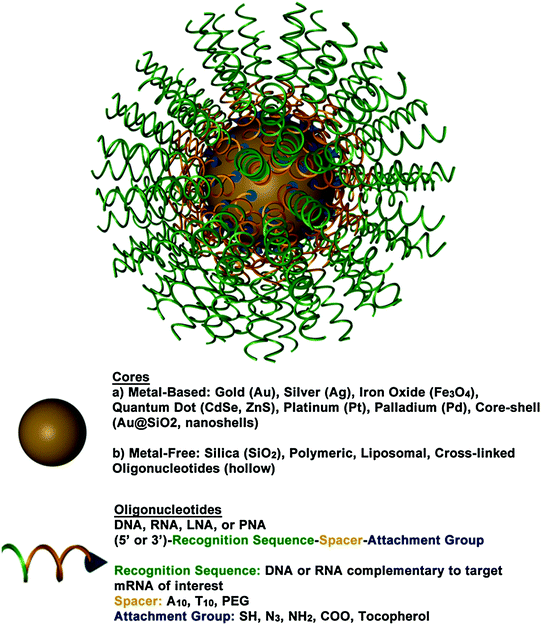

Fig. 2
A 3D drawing of a spherical nucleic acid (SNA). SNAs consist of densely functionalized and highly oriented nucleic acids on the surface of a nanoparticle. Because the properties of SNAs are derived from the shell of nucleic acids, many different cores can be used, such as metal nanoparticles (Au, Pt, etc.), liposomes, and polymers. SNAs can even be core-free. Adapted with permission from Cutler et al. [33]. Copyright 2012. American Chemical Society
1.4 Spherical Nucleic Acids (SNAs)
SNAs, typically composed of densely functionalized and highly oriented nucleic acids on nanoparticle cores, represent an emerging class of therapeutics for diseases, including many forms of cancer, because they are capable of overcoming the limitations of traditional oligonucleotide delivery methods and provide an alternative path to gene regulation (Fig. 3) [33, 34]. SNAs are single-entity agents that exhibit unique chemical and physical properties in biological environments. They are readily taken up by almost any type of cell (over 60 tested to date) in high quantities without the use of ancillary transfection reagents (>106 nanoparticles per cell) [35] through caveolae-mediated endocytosis initiated by recognition through class A scavenger receptors (SR-A) [36, 37]. They elicit a minimal immune response (i.e., 25-fold reduced immune response compared to delivery by cationic carriers) [38, 39] and exhibit increased stability compared to free oligonucleotides in solution [40–43]. These properties stem from the dense shell of highly oriented nucleic acids presented at the surface of these structures [33, 44, 45]. The fact that SNAs facilely enter cells without causing a significant immune response makes them ideal for local delivery, such as through the skin; however, the fact that they are nonspecifically picked up by nearly all cells will need to be addressed for systemic applications.
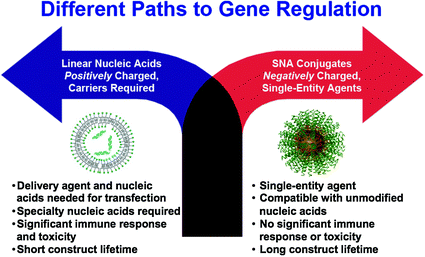

Fig. 3
SNAs offer a different paradigm for gene regulation, where negatively charged nucleic acids do not need to be precomplexed with synthetic positively charged carriers to enter cells and cause gene regulation. If the nucleic acids are densely oriented at the nanoscale, they enter cells in high numbers, exhibit nuclease resistance, show no apparent toxicity, and do not activate the innate immune response. Reproduced with permission from Cutler et al. [33]. Copyright 2012. American Chemical Society
The composition of SNAs is highly tailorable, making them an ideal therapeutic platform because they can be tuned to meet the needs of a given application. SNAs can be composed of a variety of oligonucleotides (e.g., DNA, siRNA, microRNA, peptide nucleic acid (PNA), or locked nucleic acid (LNA)) and a variety of different types of nanoparticle cores, such as gold (Au) [34], silver (Ag) [46], iron oxide (Fe3O4) [47, 48], quantum dots (CdSe, CdSe/ZnS) [49, 48], platinum [48], silica (SiO2) [50], core-shell (Au@SiO2) [50], and liposomes [51] typically ranging in size from 10 to 50 nm. Coreless versions of these structures can also be made that display the same useful properties as the core-filled structures, emphasizing the concept that the properties of SNAs stem from their densely functionalized and highly oriented nucleic acid shell and not from the nanoparticle core [51–54]. Some types of the hollow SNAs that have been synthesized thus far include those consisting of cross-linked oligonucleotides [53], DNA-block copolymer micelles [55, 56], infinite coordination polymers [52], metal organic frameworks [54], and liposomes [51]. Hollow SNAs, such as the liposomal SNAs, represent an exciting new class of metal-free SNAs that can be useful in gene regulation, and their potential is only beginning to be realized [51]. The liposomal SNAs have some exciting advantages over conventional liposomal structures, as the oligonucleotide cargo is arranged on the surface of the liposomal entity and thus stabilizes liposomes in the sub-100 nm range. Other synthetic advances in SNA development include the ability to attach RNA to DNA-based SNAs via enzymatic ligation to create RNA-DNA hybrid SNAs [57]. These structures can be used to regulate gene expression in a manner similar to other types of SNAs, and they are more cost-effective to synthesize than SNAs composed solely of RNA oligonucleotides. SNAs can also be backfilled with a variety of surface passivating molecules, such as polyethylene glycol (PEG) [40, 45] or oligoethylene glycol (OEG) [41, 45], which have been known to improve colloidal stability, increase circulation time, and reduce protein adsorption [58, 59].
1.5 Applications of SNAs in Cancer Research and Treatment
The ability to tune the oligonucleotide sequence of SNAs is extremely powerful in the development of SNAs as cancer therapeutics. For example, treatment with SNAs in vitro has resulted in gene knockdown of model targets, such as luciferase [41] and enhanced green fluorescent protein (eGFP) [42, 50], as well as targets involved in cancer cell growth and proliferation, such as HER2 (an oncogenic receptor tyrosine kinase (RTK) responsible for development and progression of cancers, in particular breast cancers) [51, 60], Bcl2L12 (a GBM oncoprotein and potent inhibitor of effector caspases and p53) [45], and epidermal growth factor receptor (EGFR; a RTK that is important for maintaining epidermal homeostasis and a potent oncogene in several cancers when overexpressed or mutated [61] both in vitro and in vivo). Because the oligonucleotide sequence can be designed to target virtually any mRNA of interest, we have only begun to scratch the surface of the potential of SNAs as cancer therapeutics; SNAs could theoretically be used to target any disease with a genetic basis, including many forms of cancer. To illustrate this versatility, we will highlight three applications below in which SNAs are used to treat cancer. First, we will highlight how SNAs can be used in the treatment of glioblastoma multiforme (GBM), the most prevalent and aggressive form of primary central nervous system malignancies. Then, we will discuss how SNAs can be delivered topically to regulate EGFR in the treatment of hyperproliferative skin disorders and skin cancer. Finally, we explore SNAs as multifunctional therapeutic agents, where drug conjugation to the SNA results in the simultaneous delivery of oligonucleotides and drugs. These structures are being evaluated for the treatment of prostate and breast cancer.
2 SNAs for the Treatment of Glioblastoma Multiforme (GBM)
2.1 Bcl2L12-Targeting siRNA SNAs
Due to the unabated growth of GBM tumors and their extensive resistance to therapies, only 3–5 % of patients survive longer than 3 years postdiagnosis [62]. Most therapeutics tested to treat GBM do not penetrate the blood–brain barrier/blood–tumor barrier (BBB/BTB) and therefore are extremely ineffective [63]. However, given that SNAs are rapidly taken up by scavenger receptors, including those found on the surface of endothelial cells of the BBB/BTB, SNAs merited investigation as a therapeutic platform that could cross the BBB/BTB and pervasively penetrate glioma tissue [64, 65]. To preclinically evaluate SNAs for the treatment of GBM, Jensen et al. utilized an in vitro co-culture model of the human BBB, consisting of human primary brain microvascular endothelial cells (huBMECs) and human astrocytes [45]. The authors designed SNAs consisting of a 13 nm gold nanoparticle core conjugated to thiolated siRNA duplexes targeting the GBM oncogene Bcl2L12, an effector caspase and p53 inhibitor overexpressed in the vast majority (>90 %) of GBM tumors [66–70]. By labeling these SNAs with a fluorescent Cy5.5 dye and employing fluorescence microscopy, the authors demonstrated that Bcl2L12-targeting SNAs (L12-SNAs) were able to undergo transcytosis through the huBMEC layer and enter human astrocytes (Fig. 4a) [71–73]. Consistent with the previous reports of SR-A-mediated uptake of SNAs [36, 37], this BBB-penetrating capacity was abolished when polyinosinic acid (Poly-I), which blocks SR-A-dependent SNA uptake and likely mediates transcytosis, was added prior to SNA treatment. Next, BBB and glioma tissue penetration was evaluated in vivo in both healthy and glioma-bearing mice. In conjunction with Cy-5 dye, gadolinium (GdIII) was conjugated to SNAs to visualize and quantify their tissue penetration; the biodistribution of these SNAs was evaluated via inductively coupled plasma-mass spectrometry (ICP-MS), magnetic resonance imaging (MRI), and confocal fluorescence microscopy. GdIII-SNA conjugates were prepared from alkyne-modified DNA thymine (dT) nucleotides and azide-labeled GdIII complexes through click chemistry. Following local administration, both 3D reconstruction of MRI images and confocal fluorescence demonstrated extensive intratumoral dissemination by SNAs (Fig. 4b). ICP-MS further validated these results, showing a 10-fold higher accumulation of SNAs in tumor versus nontumor brain regions, possibly due to the enhanced permeation and retention (EPR) effect [74]. Following tail vein injection of Cy5.5-labeled L12-SNAs, in vivo imaging system (IVIS) quantification of radiant intensities showed a 1.8-fold higher accumulation of SNAs in GBM-xenograft-bearing mice compared to sham GBM-inoculated mice (Fig. 4c). Furthermore, systemically delivered L12-SNAs successfully neutralized Bcl2L12 expression, increased intratumoral apoptosis, reduced tumor burden, and increased survival. Systemically administered L12-SNAs did not induce inflammatory cytokines, cause any changes in blood chemistry and complete blood counts, or elicit changes in histopathology compared to saline or control SNAs. With no evidence to date of toxicity and promising in vivo results thus far, L12-SNAs represent a promising construct for GBM treatment that is headed toward early clinical testing.
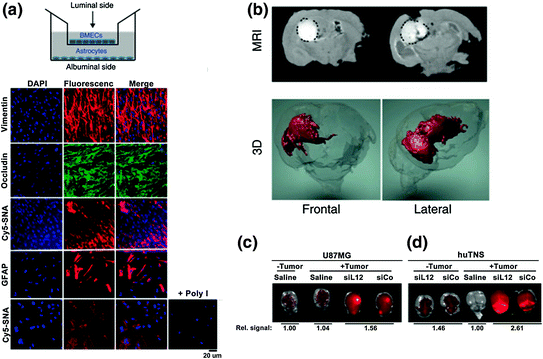

Fig. 4
BcL2L12-SNAs in treatment of glioblastoma multiforme. SNAs cross the BBB/BTB and selectively accumulate in glioma tissue. a Noncontact in vitro BBB model using a co-culture of human primary brain microvascular endothelial cells (huBMECs) and human astrocytes. Representative confocal fluorescence microscopy images demonstrate Cy5.5-SNA (red) distribution in endothelial and astrocytic cells. Endothelial and astrocytic cells stained positively for occludin (a marker for tight junctions) and glial fibrillary acidic protein (GFAP), respectively. The cytoplasm is stained with DAPI and the nuclei stained with anti-vimentin; b Magnetic resonance (MR) images of tumor-bearing mouse brains injected intracranially with SNAs-GdIII. Two representative coronal sections imaged 24 h after SNAs-GdIII injection (upper panel) show localization of SNAs-GdIII within the intracerebral lesion. GdIII signal is white and outlined with a black dotted line. Also shown is the corresponding three-dimensional (3D) reconstruction of MR images (GdIII signal in red); c and d IVIS analysis of brains with or without human glioblastoma-astrocytoma (U87MG) (c) or human tumor neurospheres (huTNS) (d) tumors 48 h after systemic delivery of saline or Cy5.5-SNAs. SNA accumulation is indicated by increased fluorescence (yellow). Quantification of radiant efficiency is shown as relative signal amount under the images. SiCo = scrambled control sequences. Adapted with permission from Jensen et al. [45]. Copyright 2013. Science Translational Medicine
2.2 Delivery of Therapeutic miRNA Using miR-182 SNAs
miRNAs have been shown to be important regulators of GBM pathogenesis and therapeutic susceptibility [75]. Genomic studies have characterized miRNA-controlled signaling pathways, which include critical growth and survival pathways, such as receptor tyrosine kinase (RTK)-phosphoinositide 3-kinase (PI3K)-phosphatase and tensin homolog (PTEN), retinoblastoma (Rb), B-cell lymphoma 2 (Bcl-2), and tumor protein p53 (p53) signaling pathways [76, 77]. Given the global overexpression of Bcl2L12, its roles in the pathogenesis of GBM, and its involvement in therapy resistance, the Kessler, Peters, Mirkin, and Stegh labs sought to identify miRNAs that control the expression of Bcl2L12 in GBM [78]. In silico studies of GBM samples from the multidimensional Cancer Genome Atlas (TCGA) dataset (http://cancergenome.nih.gov/dataportal/) were designed to discover miRNAs with expression levels negatively correlated with Bcl2L12 mRNA levels (Cancer Genome Atlas Research [79]. From these studies, miR-182 was identified as a potential miRNA candidate that regulates Bcl2L12 expression.
The authors demonstrated that miR-182 acts as a tumor suppressor in GBM by not only controlling the expression and activity of Bcl2L12, but also levels of the RTK c-Met and the transcription factor hypoxia-inducible factor 2α (HIF2α). To harness miR-182-related tumor suppressive functions as a therapeutic in vitro and in vivo, the authors synthesized SNAs functionalized with mature miR-182 sequences (182-SNAs). Treatment of glioma cells with 182-SNAs in vitro was shown to potently decrease Bcl2L12 and c-Met protein levels compared to control SNA-treated cultures, while substantially increasing apoptotic responses and reducing cellular growth. The authors then evaluated 182-SNAs in mice bearing orthotopic GBM xenografts in vivo. In 182-SNA-treated mice compared to control SNA-treated mice, average tumor weights were reduced, and 182-SNA-treated mice experienced significantly prolonged survival relative to control SNA-treated mice.
Taken together, 182-SNAs were shown to effectively decrease Bcl2L12 and c-Met protein levels, enhance apoptotic responses to chemotherapy, drastically reduce tumor burden, and extend survival of GBM-xenograft-bearing mice. Coupled with the absence of any observable side effects or toxicity, 182-SNAs represent a novel platform for delivering therapeutic miRNAs in GBM.
3 Topical Delivery of SNAs to Regulate Epidermal Growth Factor Receptor (EGFR)
In order to suppress cancer-causing genes in the skin using oligonucleotides, the oligonucleotide must pass the epidermal barrier. One of the greatest challenges associated with topical drug delivery is the design and synthesis of materials that can pass through this barrier [80–82]. In skin, topical delivery is the desired route for delivering agents that can regulate gene suppression because the skin is easily accessible and because topical delivery reduces the risk of systemic side effects. Therefore, an agent that can deliver oligonucleotides through the epidermal barrier with relatively little cytotoxicity would be ideal. Recently, it has been shown that SNAs can be delivered topically in a commercial moisturizer or phosphate buffered saline (PBS) solution to target EGFR, an important gene for epidermal homeostasis and potent oncogene that is frequently overexpressed or mutated in cancer [83, 84]. SNAs were able to penetrate through hairless mice and human skin equivalents without any clinical or histological evidence of toxicity [39]. The Mirkin and Paller labs first measured the uptake of SNAs in normal human keratinocytes (hKCs) because they are notoriously difficult to transfect [85]. ICP-MS revealed that SNA uptake is five times higher in hKCs as compared to HaCaT (spontaneously immortalized hKCs) or HeLa cells [39]. Although the underlying mechanism for the high cellular uptake in hKCs is still under investigation, these data show the potential of SNAs as a strong candidate for topical oligonucleotide delivery. When the uptake in hKCs was visualized by confocal microscopy using nontargeting SNAs with a Cy3 dye, strong fluorescence was seen in the cytoplasm of the cells and morphological differences were not seen between untreated hKCs and SNA-treated hKCs (Fig. 5a). To determine potential off-target effects of SNAs, genome-wide expression profiling revealed only seven up-regulated genes (none were downregulated); in contrast, 427 genes were up- or down-regulated in the case of lipid-based siRNA delivery using the DharmaFECT1™® transfection reagent. This is a promising result because SNA toxicity should be relatively low since there are virtually no off-target effects.
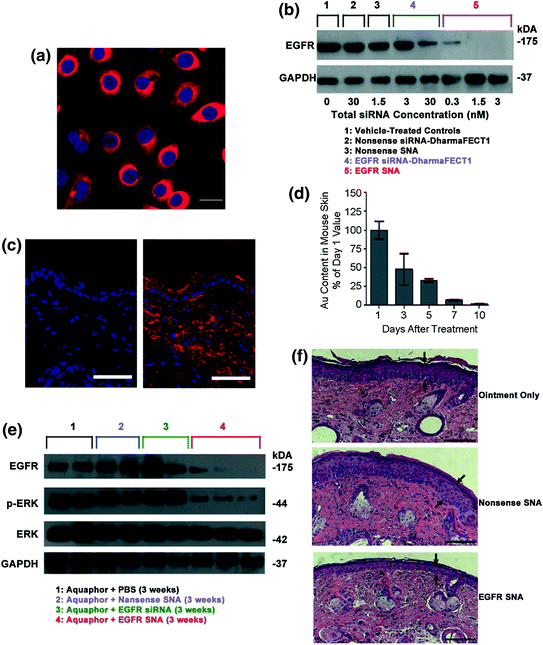

Fig. 5
Topical delivery of SNAs through human and mouse skin. a Uptake of Cy3-labeled nonsense SNAs (red) in the cytoplasm of approximately 100 % of the primary human keratinocytes (hKCs) after 24 h incubation. The nuclei were stained blue with Hoechst 33343. Scale bar, 20 μm; b Western blot showing epidermal growth factor receptor (EGFR) protein levels for hKCs treated for 48 h. Note the greater suppression by 0.01 nM EGFR SNAs (equivalent to 0.3 nM siRNA) as compared with the 30 nM EGFR siRNA delivered with DharmaFECT1™ at 60 h; c Mouse (SKH1-E) skin treated topically with 1:1 Aquaphor® only (left) or with 50 nM Cy5-labeled (red) SNAs dispersed in the 1:1 Aquaphor® (right). The SNAs are seen in the cytoplasm of epidermal cells and the dermis 3 h after application. DAPI-stained nuclei in blue. Scale bars, 100 μm; d Mouse skin was treated daily for 3 days with nonsense SNAs and analyzed by ICP-MS for gold content. The gold content in mouse skin progressively decreases after cessation of topical treatment; 10 days after the final treatment, only 2 % of the original gold content remains (n = 3 at each time point); e The protein expression of EGFR was nearly eliminated in the EGFR SNA-treated group, whereas the downstream phosphorylation of ERK was inhibited by 74 %; total ERK expression remained constant; f The mean thickness of EGFR SNA-treated skin was 40 % less than that of control-treated skin (P < 0.001), as measured by computerized morphometry. Epidermal thickness was measured from the top of the stratum granulosum to the basement membrane (arrows) at three equidistant sites. Adapted with permission from Zheng et al. [39]. Copyright 2012. Proceedings of the National Academy of Sciences of the United States
After confirming that SNAs enter hKCs and cause relatively little immune response, the potential for EGFR mRNA and protein knockdown was assessed. Western blot analysis showed that there was greater EGFR suppression by 0.3 nM siRNA delivered by SNA than 30 nM siRNA delivered by DharmaFECT1™ in hKCs (Fig. 5b). In fact, total suppression of EGFR, as seen by Western blot, was observed with incubation of 1.5 nM total siRNA delivered by SNAs, something that was not seen even at 30 nM siRNA delivered by DharmaFECT1™, thus demonstrating the potency of oligonucleotide delivery via SNAs.
The authors then investigated the potential of SNAs to penetrate mouse skin. In both SKH10E hairless mice and hair-bearing C57BL/6 J mice that were shaved 24 h before treatment, SNA penetration through the stratum corneum and into the epidermis and dermis was seen in as few as 3 h after a single SNA dose (Fig. 5c). Furthermore, after treating SKH1-E hairless mice daily for 3 days with nontargeting SNAs and monitoring the gold content in skin for 10 days post-treatment, it was found that only 2 % of the initial gold was still present in the skin (Fig. 5d). In summary, SNAs were shown to penetrate the skin of two different mice strains and subsequently clear the skin by 10 days post-treatment.
The next step was to investigate the efficacy of EGFP suppression in mouse skin. Western blot analysis showed that the protein expression of EGFP was nearly eliminated and the downstream phosphorylation of ERK was inhibited by 74 % from the application of SNAs in Aquaphor®; Aquaphor® alone, nontargeting SNAs, and free siRNA did not have an effect on EGFP expression (Fig. 5e). Furthermore, the decrease in EGFP expression was accompanied by a 74 % decrease in the phosphorylation of downstream extracellular signal-regulated kinases (ERK1/2), demonstrating the specificity of EGFR knockdown (total ERK1/2 expression remained constant). To confirm the phenotypic effect of EGFR knockdown on mouse skin, computerized morphometric analysis of histological sections of mouse skin treated with EGFR SNAs showed an almost 40 % reduction in thickness compared to the mouse skin treated with Aquaphor® alone or nontargeting SNAs (Fig. 5c). Similar results were seen with human skin equivalents. Taken together, these data demonstrate the ability of SNAs to knockdown a specific gene target in vivo, which causes a specific biological response with minimal off-target effects. This study lays the groundwork for SNAs to be utilized for topical oligonucleotide delivery with vast potential in the treatment of skin diseases and disorders, such as metastatic melanoma and psoriasis.
4 Multifunctional SNAs
Thus far, we have discussed SNAs that enter cells and tumor tissues and perform a single function (e.g., regulate gene expression via the RNAi or antisense pathway). However, SNAs that can perform multiple therapeutic, diagnostic, targeting, and imaging functionalities simultaneously within a cell can also be synthesized. Using solid-phase DNA synthesis, modified phosphoramidites can be used to add specific chemical functional groups onto oligonucleotides; and these functional groups can be used as handles to attach drugs, small molecules, or antibodies of interest to these oligonucleotides. When these modified oligonucleotides are formulated as SNAs, the SNA structure allows for the cellular entry of the conjugate and the oligonucleotides allow for the regulation of gene expression, while the small molecule drug or contrast agent that is appended to the oligonucleotides that comprise the SNAs, for example, can be used as an additional therapeutic component or imaging modality, respectively.
4.1 SNA-Drug Conjugates for Drug Delivery
Cisplatin and carboplatin are widely regarded as effective treatments for testicular and ovarian cancers, and these drugs have also been utilized for the treatment of bladder, cervical, head and neck, esophageal, and small cell lung cancer [86, 87]. However, many of the synthetic delivery systems used for cisplatin and carboplatin are associated with systemic toxicity [88]. Because SNAs have been shown to enter cells in high quantities [35] without provoking a significant immune response [38], they are a promising platform for the delivery of these and other platinum (Pt) compounds. To this end, the Lippard and Mirkin labs collaborated to synthesize a DNA oligonucleotide with a terminal dodecyl amine to which the Pt (IV) pro drug (c,c,t-{Pt(NH3)2Cl2(OH)(O2CCH2CH2CO2H}) was conjugated via amide linkages (Fig. 6a) [89]. Platinum atomic absorption spectroscopy (AAS) showed that 98 % of the DNA amines on the SNA were conjugated to platinum. The mechanism of action for Pt (IV) complexes requires that they are reduced to cytotoxic Pt (II) in a reducing environment, such as inside cells or blood [90]. Electrochemical studies confirmed that the conjugation of a Pt (IV) payload to the SNA did not significantly alter the reduction potential of the complex, making it likely that the axial ligands of the Pt (IV) complex could be removed once it entered a reducing environment. It was also confirmed that the SNAs still entered HeLa cervical cancer cells with the Pt (IV) prodrugs attached (Fig. 6b), and specifically colocalized with microtubules (Fig. 6c). Finally, the efficacy of the SNA-bound Pt (IV) prodrug was assessed in four different cell lines. For A549 lung epithelial cancer cells in particular, the conjugation of the Pt (IV) prodrug to the SNA resulted in superior killing efficiency, with a half maximal inhibitory concentration (IC50) of 0.9 μM, compared to an IC50 of 11 μM for free cisplatin. Therefore, it appears that the Pt (IV) prodrug is more effective when conjugated to DNA on the SNA surface. Future work will include using the oligonucleotide, whether it is DNA or RNA, to knockdown gene expression, which, in conjunction with drug delivery, should render these constructs even more effective.
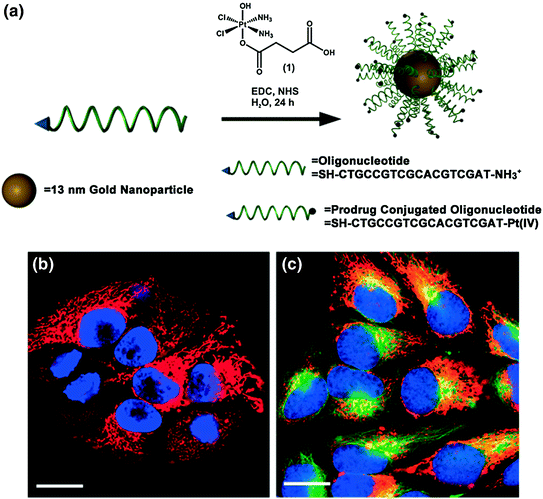

Fig. 6
Drug and oligonucleotide dual therapy. a Scheme for the synthesis of Pt (IV) terminated SNAs; b Live cell imaging of HeLa cells upon incubation with platinum-tethered Cy5-SNAs for 12 h; c Co-localization of the particles with the cytoplasmic microtubules. Hoechst 33342 was used for nuclear staining. Scale bars, 20 μm. Adapted with permission from Dhar et al. [89]. Copyright 2009. American Chemical Society
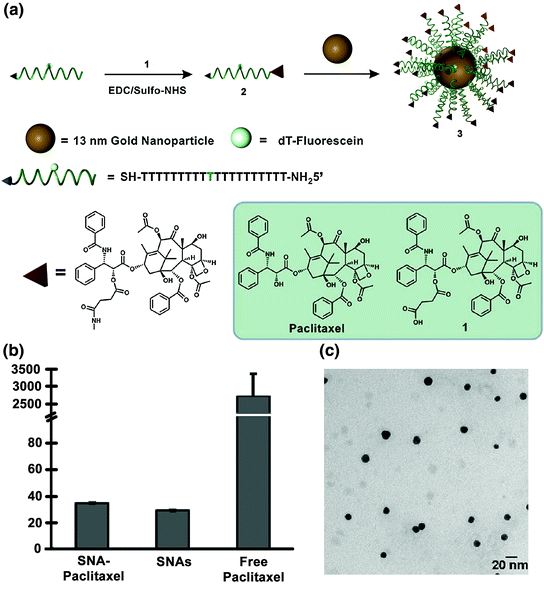
The Ho and Mirkin labs employed a similar strategy with the chemotherapeutic Paclitaxel [91]. Paclitaxel is used to treat cancers such as ovarian, breast, and nonsmall cell lung cancers [92, 93]. Paclitaxel is challenging to deliver because of its low aqueous solubility; cells also acquire chemoresistance toward this drug and it often causes harmful side effects [94]. Therefore, it was hypothesized that Paclitaxel’s conjugation to SNAs may increase its aqueous solubility and perhaps decrease the associated side effects.
Zhang and co-workers conjugated a thiolated oligonucleotide containing a terminal Paclitaxel group to 13 nm gold nanoparticles (AuNPs) (Fig. 7a; compound 3) [91]. This step was accomplished by modifying Paclitaxel molecules with succinic anhydride groups to form a Paclitaxel carboxylic acid derivative (compound 1), which was conjugated to DNA oligonucleotides with a terminal amine group using EDC/sulfo-NHS chemistry (compound 2). The average number of Paclitaxel molecules per gold nanoparticle was measured to be 59 ± 8. It is interesting to note that while free Paclitaxel is not soluble in phosphate buffered saline (PBS) at a 5 μM concentration, the SNA-Paclitaxel conjugates remain well dispersed in PBS, as confirmed by dynamic light scattering (DLS) (Fig. 7b) and transmission electron microscopy (Fig. 7c). Free Paclitaxel has a maximum solubility of 0.4 μg/mL in aqueous solution [95] and the SNA-Paclitaxel conjugate exhibits a maximum solubility for Paclitaxel of 21.35 μg/mL, a greater than 50-fold enhancement in drug solubility. Confocal microscopy confirmed the internalization of fluorophore-labeled SNA-Paclitaxel conjugates in MCF7 human breast adenocarcinoma cells and MES-SA/Dx5 human uterine sarcoma cells after a 6 h incubation. A terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay [96] determined that DNA fragmentation and apoptosis was induced by Paclitaxel. It was shown that Paclitaxel remained active when bound to SNAs and that the SNA-Paclitaxel conjugates have the potential to overcome Paclitaxel resistance in cells.