Although the question is not settled, there is a growing consensus in the literature that minimally invasive techniques may be employed for excision of primary adrenal malignancy in appropriately selected cases. It also is now becoming evident that laparoscopy provides similar long-term results to open adrenalectomy when employed for solitary metastases to the adrenal.15 Appropriate planning and patient selection are imperative. If preoperative imaging suggests invasion into surrounding structures or vena caval thrombus, one should proceed with open resection. Principles of resection that apply in open surgery must be maintained in laparoscopy, including preservation and dissection of tissue planes and avoidance of fracture or rupture of the lesion. The specimen should be removed from the body in an impermeable retrieval bag to avoid exposure of the port site. It is obvious that the surgeon must be experienced in both laparoscopy and endocrine surgery. Because malignancy may be undiagnosed prior to resection, all of these principles should be applied to laparoscopic and open adrenalectomy in general, with particular attention in large or suspicious lesions.
There remains debate in the literature regarding how large of a lesion should be approached laparoscopically, both for oncologic and technical reasons. A large lesion makes the operation more challenging. Most series reporting resection of large masses, including those in Table 110-1, emphasize the lateral transperitoneal technique, which allows the greatest visualization and operative space. Even with this approach, however, visualization can be a challenge in large lesions. Large lesions also may have aberrant vascular supply, thus leading to potential for operative bleeding. While lesions as large as 15 cm have been resected laparoscopically,17 the appropriate size restrictions for laparoscopic adrenalectomy has not been universally agreed upon. Multiple series have now been published of laparoscopic adrenalectomy for lesions over 6 cm.15,18–21 Hospital length of stay, operative blood loss, and overall morbidity are not necessarily worsened following laparoscopic resections of large lesions. Rosoff’s review concludes that tumor size alone should not preclude laparoscopic approaches, but that larger lesions should be managed by experienced laparoscopic surgeons who also are adept at the open procedure, should conversion be required. The review also emphasizes the importance of adhering to the oncologic principles discussed earlier.22 Previous upper-abdominal surgery such as nephrectomy, splenectomy, or hepatic resection is not an absolute contraindication to laparoscopy but does make the procedure more difficult. This could necessitate conversion to an open procedure, potentially in an urgent fashion if difficult dissection leads to operative bleeding. Retroperitoneal laparoscopic approach has been reported to circumvent these problems, but this procedure is technically challenging and not widely practiced.
Robotic surgery is a fairly recent development in adrenal surgery. Robotic computer-assisted telemanipulation systems were developed to overcome some of the limitations of standard laparoendoscopic techniques and to facilitate surgeon hand motions in limited operating spaces. The surgeon operates from a remote console, and hand motions are reproduced in scaled proportion through robotically controlled microwrist instruments inserted through the body wall. Purported advantages of robotic-assisted laparoscopic surgery over conventional laparoscopy include hand motions intuitive even to the nonlaparoscopic surgeon,23 seven degrees of freedom of motion (compared to four with standard laparoscopy), three-dimensional image projection, tremor suppression, motion scaling, and the potential to perform remote “telesurgery.”
Robotic approaches have been applied in a wide variety of urologic, cardiothoracic, gynecologic, and general surgical procedures. The first report of robotic adrenalectomy was by Piazza et al. in 1999.24 Hanly and colleagues reported 30 robotic adrenalectomies without any conversions to open procedures.25 They felt that the robotic system permitted improved identification and control of the multiple adrenal arteries. Although all other reports of robotic adrenalectomy have been for benign lesions, a robotic resection of an 8-cm adrenal lesion that proved to be adrenocortical carcinoma with clean margins has now been reported, with good early results.26
Several reports have compared robotic to laparoscopic adrenalectomy. No difference was found in perioperative quality of life measures between laparoscopic and robotic adrenalectomy.27 An earlier randomized series compared one hospital’s first 10 robotic adrenalectomies to 10 laparoscopic adrenalectomies28 and reported that laparoscopy was superior to robotics in terms of morbidity and cost. More recently, a retrospective review of prospectively collected data compared 50 patients undergoing robotic unilateral adrenalectomy to 59 patients undergoing laparoscopic unilateral transperitoneal adrenalectomy.29 They found that the robotic approach was associated with lower blood loss but longer operative times early in their series. Interestingly, they reported a learning curve of 20 robotic adrenalectomies, after which the difference in operative time was absent. Furthermore, operative times were not affected by obesity or large tumors in the robotic group, while these factors led to significantly longer operative times in the laparoscopic group. Conversion rate, morbidity, and hospital stay were similar between groups.
Limitations of robotics and robotic adrenalectomy include the high cost of the permanent equipment and the semi-reusable instruments, the absence of good data for benefit compared to standard laparoscopy, the learning curve, and the lack of tactile feedback to the surgeon. While the role and future of this technology remain to be determined, one expects that the lessons and advances from robotics will allow further advances in adrenal surgery.
Preoperative History and Evaluation
Patients with functional adrenal tumors may present with characteristic metabolic disorders. Patients with nonfunctional masses or malignancies should undergo routine evaluation for general anesthesia, which is required for either minimally invasive or open adrenalectomy. In the patient with a functioning adrenal mass, specific attention may be needed to correct any metabolic abnormality. The patient with an aldosterone-secreting tumor, hypertension, and significant hypokalemia will require medical correction. Potassium replacement with oral supplementation is usually sufficient. Hypertension treatment can be undertaken with a variety of medications, including calcium channel blockers and angiotensin-converting enzyme (ACE) inhibitors. Hyperglycemia occurs in as many as 15% of these patients, and they may require insulin treatment. The patient presenting with Cushing’s syndrome and a unilateral adrenal mass may have suppression of the contralateral adrenal. Therefore, preoperative preparation with stress-dose steroids (e.g., 100 mg of hydrocortisone) is generally indicated. This dose is also adequate for patients undergoing bilateral adrenalectomy for persistent ACTH-secreting syndromes. The former group of patients will require postsurgical steroid tapering, whereas the latter group will require lifelong exogenous steroid replacement.
The patient with pheochromocytoma deserves particular attention (see Chapter 109). Once this diagnosis is made, medical management must address hypertension, decreased intravascular volume, and possible cardiac arrhythmias. The use of phenoxybenzamine, a long-acting α-adrenoceptor blocker, is usually suggested for 1 to 3 weeks prior to surgery (see also Chapter 109). More recently, some groups have reported the use of preoperative blockade with selective postsynaptic α1-adrenergic receptor antagonists such as prazosin hydrochloride or doxazosin. Such selective agents are thought not to produce reflex tachycardia and have a shorter half-life. These agents may avoid postoperative hypotension that are associated with the longer-acting drugs. These are important considerations, yet phenoxybenzamine remains a standard and time-tested preoperative blockade. The use of calcium channel blockers or ACE inhibitors has also gained acceptance. Because these medications are given in the preoperative period, attention should be given to oral salt and fluid intake to address expansion of intravascular volume prior to surgery. This preoperative regimen must be closely watched. During this time, patients will retain fluid and demonstrate side effects of the medication, possibly including sinus discomfort and orthostatic hypotension. Additionally, a small subset of patients may have arrhythmias which may require β-adrenoceptor blockade. Effective β-blockers include propranolol and nadolol.30 Our group has had excellent experience with metyrosine, 1 to 4 g/day for 10 to 14 days prior to surgery, in addition to phenoxybenzamine. Metyrosine is a tyrosine hydroxylase inhibitor and is very effective in reducing blood pressure; it is associated with fatigue and sinus discomfort. Patients need to be instructed carefully about its side effects.
Preoperative imaging must be evaluated by expert interpretation and also should be studied by the operating surgeon. CT and MRI form the cornerstones of workup and preoperative diagnosis. These films must be of adequate technique and quality to allow investigation of the retroperitoneum and the contralateral adrenal, as well as any other anatomy relevant to the diagnostic workup (e.g., the lung fields, in the case of metastasis). In the case of aldosterone excess, the diagnosis of a solitary adrenal mass must be made to secure the diagnosis of Conn’s syndrome. If both adrenals are not investigated, occasional macronodular bilateral hyperplasia might present with a unilateral nodule that, although appearing to be dominant, might draw attention away from less discrete nodularity in the contralateral gland (Figs. 110-1, 110-2). This situation might require bilateral adrenal vein sampling for aldosterone levels (see Chapter 107). In the case of the pheochromocytoma, multicentric tumors in the retroperitoneum and the contralateral adrenal must be excluded. Any suspicious masses may require MIBG radionuclide study or T1- and T2-weighted MRI scanning (Fig. 110-3).
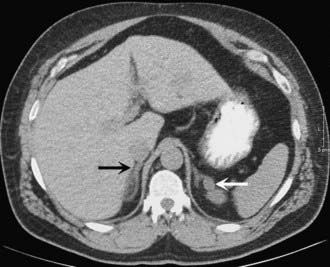
FIGURE 110-1. CT scan of 36-year-old female with hypertension and hypokalemia. Initial workup demonstrated a 1.2-cm left adrenal mass (white arrow). However, subsequent targeted imaging suggests a mass in the right adrenal (black arrow), raising the question of bilateral macromodular hyperplasia.
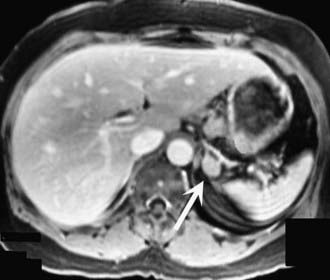
FIGURE 110-2. MRI scan shows an enlarged left adrenal mass in a patient with Conn’s syndrome (arrow). Right adrenal is seen behind the vena cava and is normal size.
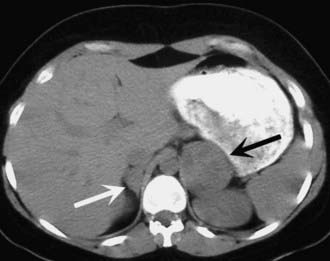
FIGURE 110-3. A 42-year-old female with hypertension and MEN2 syndrome. CT scan reveals bilateral adrenal masses, which were pheochromocytomas.
The experienced endocrine surgeon should personally investigate any or all preoperative laboratory and imaging studies, using local or regional expertise in imaging. Repeat studies are warranted if there is any doubt as to the diagnosis, since operative and perioperative management vary with different pathologies.
Surgical Anatomy, Approaches, and Techniques
The surgical approach to the adrenal requires a complete understanding of the surgical anatomy and the available preoperative and intraoperative options. One of several approaches can be chosen. The open technique allows access to both adrenals and the retroperitoneum (transabdominal). The lateral techniques (open, laparoscopic, or robotic) allow a more focused approach to unilateral adrenal masses and to retroperitoneal areas and kidney. The posterior approach (open or laparoscopic) allows fairly limited access to the adrenal gland on either side but is thought to be associated with fewer postoperative complications.
SURGICAL ANATOMY
The adrenal glands are retroperitoneal organs. They are slightly nodular with a firm texture, and they are surrounded by a layer of loose connective tissue. The arterial supply of both sides consists of multiple small branches that enter the superior, medial, and inferior surfaces of the gland. Venous drainage is much more constant and usually is via a single adrenal vein, with different drainage for the right and left gland.
The right adrenal gland is situated adjacent and medial to the superior pole of the right kidney. It is immediately anterior to the posterior reflection of the diaphragm and adjacent to the right and posterior border of the inferior vena cava. The right adrenal is inferior to segment VII of the liver. The arterial supply is from the inferior phrenic artery and from branches of the renal artery, lumbar arteries, and aorta. The right adrenal vein is approximately 3 mm in size; it courses medially and empties directly into the inferior vena cava. In very rare cases, the adrenal vein can course superiorly to empty into the right hepatic vein.
The left adrenal gland is also superior and medial to the superior pole of the left kidney. This position is posterior to the stomach and pancreas in the retroperitoneal area, behind the lesser sac. The medial aspect of the left adrenal gland lies along the left crus of the diaphragm and aorta. Dissection in this area will usually expose the esophagus. Like the right adrenal, the arterial supply is from the renal artery, inferior phrenic artery, lumbar arteries, and aorta. The left adrenal vein often courses inferiorly, emptying into the left renal vein. Occasionally, the left adrenal vein can course medially, joining with a phrenic vein before emptying into the renal vein. Approaching either adrenal, the surgeon must be aware that the vessels of the renal hilum are near the inferior aspect of the dissection, and that extreme care must be taken not to stray into vessels supplying the renal parenchyma.
SURGICAL APPROACHES
General endotracheal anesthetic is used in all cases. Foley catheter and gastric decompression are instituted. Depending on the approach, appropriate padding and support must be used to protect weight-bearing areas and prevent compression or stretch neurapraxic injury to areas such as the brachial plexus and peroneal nerve. Antibiotics are administered 30 minutes prior to skin incision. Compression boots or stockings are instituted for mechanical thromboprophylaxis prior to anesthetic induction.
Open Transabdominal (Anterior) Approach
Until 20 years ago, this was the classic surgical approach, particularly in the case of familial pheochromocytoma. It allows access to both adrenals and full exploration of intraabdominal and retroperitoneal extraadrenal deposits of tumor. The midline or bilateral subcostal incision is employed, allowing access to the peritoneal cavity with the patient positioned supine. The right adrenal gland is approached by mobilizing the hepatic flexure of the colon and right lobe of the liver. A generous Kocher maneuver is performed, mobilizing the duodenum medially (Fig. 110-4). In this manner, a full view is gained of the vena cava from the level of the right renal vein to the diaphragm. The adrenal gland is usually dissected from a lateral position, mobilizing it off of the kidney and from the retroperitoneal tissues; the gland is mobilized medially until the right adrenal vein is seen and securely ligated.
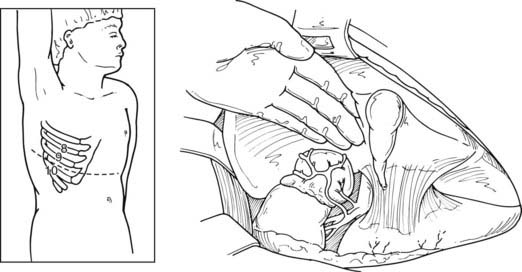
FIGURE 110-4. Approach to a right adrenal mass via open transabdominal technique. The incision can be extended laterally to a thoracoabdominal incision via the 9th-10th interspace.
(From Scott HW Jr, Oates JA, Nies AS et al: Pheochromocytoma: present diagnosis and management, Ann Surg 183:587, 1976.)
The left adrenal gland is approached by entering the lesser sac and taking down the short gastric vessels along the greater curve of the stomach. The spleen and tail of the pancreas are mobilized medially by dividing the splenocolic ligament and mobilizing the spleen and tail of the pancreas medially, thereby allowing a full view of the left retroperitoneal space. The left adrenal gland is then approached, either by (1) isolating the hilum of the left kidney and identifying the left adrenal vein or (2) superiorly, by tracing the inferior phrenic vein to the top of the adrenal. Medial dissection usually allows release of the adrenal from the aorta. Care must be used in the most inferior aspect of this dissection while identifying the adrenal vein. Additional care must be used in the space between the adrenal and kidney to avoid damage to any arterial branches coursing superiorly to the superior pole of the left kidney.
Open Lateral Approach
This approach has been used more commonly since the 1980s and before the advances in laparoscopic procedures. After inducing general anesthesia, the patient is placed in the lateral decubitus position, with the table flexed just above the anterior superior iliac spine; this accentuates the area between the costal margin and the iliac crest on the side of the lesion. The patient is rolled forward slightly, which has the added advantage of allowing the abdominal pannus to fall out of the surgical field, allowing excellent exposure of the costoiliac area. In patients with Cushing’s syndrome, this maneuver is particularly helpful. The tip of the 11th or 12th rib can be palpated fairly easily in this position even if the patient is somewhat obese. A curvilinear incision is employed on the 11th rib. The periosteum is stripped off of the rib, which is then resected at the base. Care must be used to preserve the intercostal neurovascular bundle. By incising through the medial aspect of this incision, the abdominal musculature can be divided, and the peritoneum can be pushed anteriorly and not entered. The pleura can be usually demonstrated and pushed superiorly. If the pleura is entered, it can be repaired under positive-pressure ventilation, which usually does not require a chest tube. Immediately inferior to the edge of the diaphragm is the area of perinephric fat at the superior aspect of the kidney; Gerota’s fascia is encountered here and incised. This area is entered, and by proceeding medially, the surgeon can identify either the adrenal tumor or normal-appearing cortex of the adrenal, which stands out in contrast to the surrounding fat (Fig. 110-5). These procedures can be performed through an 8- to 10-cm incision, employing self-retaining retractors to gain optimal exposure. On the left, the surgeon must be careful about vigorous retraction on the spleen; on the right, similar care must be used on the liver. The dissection of each adrenal gland proceeds as described for the anterior open approach.
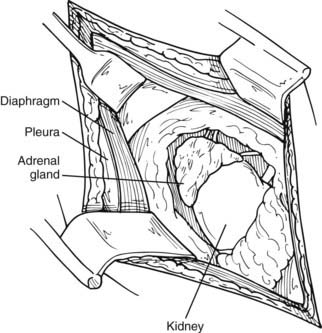
FIGURE 110-5. Lateral open approach to an adrenal mass on the right through an 11th-rib resection. Diaphragm and pleura are retracted superiorly, and Gerota’s fascia is incised, allowing exposure of the mass.
(From Scott HW, Liddle GW, Mulherin JL et al: Surgical experience with Cushing’s disease, Ann Surg 185:524, 1977.)
After completion of the adrenalectomy, the patient is taken out of the flex position on the table, and the incision is closed in layers. The intercostal bed is closed. This should be done with full view of the intercostal neurovascular bundle, so as not to injure them and cause bothersome postoperative pain. Often the intercostal nerve can be injected at this time with 0.25% Marcaine. The latissimus dorsi and abdominal muscles can then also be closed using a running suture. A chest tube is not usually required unless parenchymal damage to the lung has occurred. Every effort should be made to close any small holes in the pleura, which can occur if an 11th rib or higher approach is used.
Open Posterior Approach
Perhaps the most direct anatomic route to the adrenal glands is through a posterior approach. This is particularly true for smaller tumors. There is general agreement that this is not a preferred approach for lesions larger than 5 cm. Prior to the advent of laparoscopy, it was felt that the posterior approach allowed for the shortest hospitalization and lowest morbidity.
The patient is placed in a prone position, with the operating table in a flex position. Hyperflexion of the lower back area is achieved by positioning the table and by the use of pillows under the abdomen. In this fashion, the area between the posterior iliac crest and the posterior costal margin is accentuated. The advantage of this position is that it allows access to both adrenals. The disadvantage is very limited exposure; therefore, the incision and consequent dissection must be carefully planned. A curvilinear incision starting parallel to and about 3 inches lateral to the spine and turning sharply out over the 12th rib is preferred. This allows exposure to the latissimus dorsi muscle and the sacrospinal fascia. The latissimus dorsi and the lateral aspect of the sacrospinal are divided, and the 12th rib is identified. The periosteum is stripped along the entire length of the rib, taking care to preserve the intercostal neurovascular bundle. The rib is removed, and the bed of the rib is incised. This allows access to the retroperitoneum, exposing the perinephric fat. Superior retraction identifies the parietal pleura and the lateral arcuate ligament of the diaphragm. The pleura should be reflected upward, allowing reasonable exposure to the perinephric fat. Dissection through the fat exposes the upper pole of the kidney, which is retracted inferiorly. It is at this time that distinct limitations in visibility can be appreciated if the appropriate dissection has not been done (Fig. 110-6). The gland on either the right or left side should be identified in the perinephric fat. Superior dissection usually allows enough mobilization to gain access to the adrenal vein, coursing into the vena cava (on the right side) or coursing inferiorly into the left adrenal vein (on the left side).
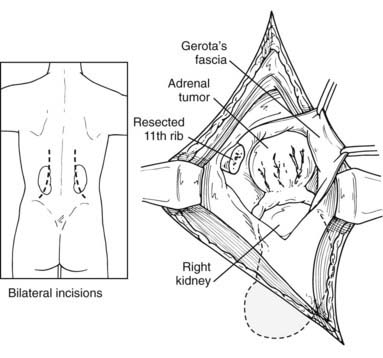
FIGURE 110-6. Posterior, transdiaphragmatic approach to adrenal mass. Bilateral incisions may be used for bilateral tumors.
(From Scott HW Jr, Foster JH, Liddle G, Davidson ET: Cushing’s syndrome due to adrenocortical tumor, Ann Surg 162:505, 1965.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








